Clinical Trial Participant Says He Suffered Heart Attack
Volunteers for a drug trial are expected to sign an ‘informed consent’ form. Every drug trial has its own independent ethics committee.
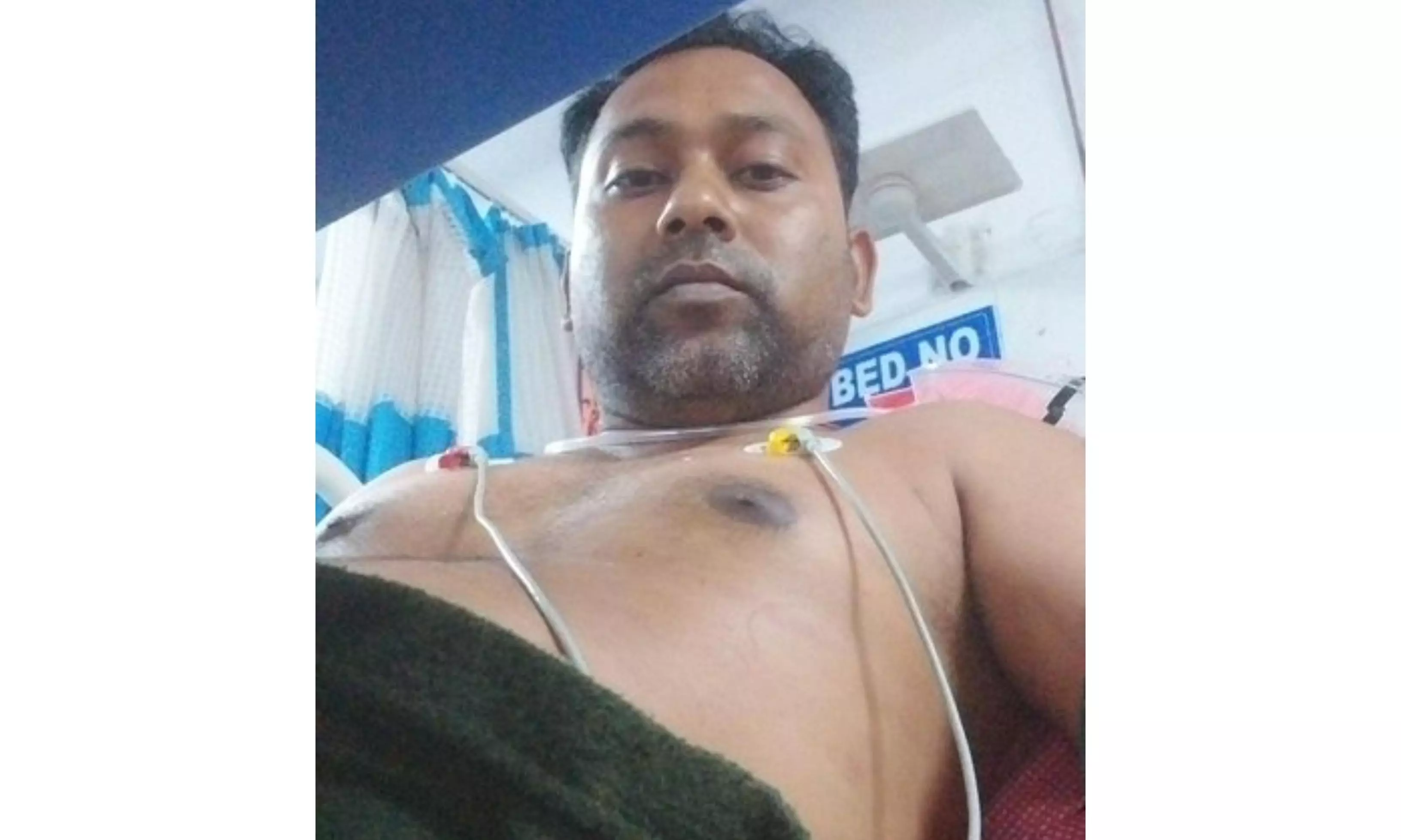
Hyderabad: @CPHydCity. @harichandanaias. @MoHFW_Telangana. @Collector_HYD. @TGMedCouncil.
These are among the X (Twitter) handles of officials that a 38-year- volunteer of an ongoing clinical trial reached out to for help after suffering a heart attack. He was administered a drug for breast cancer for which the trial was being conducted by a Hyderabad based clinical research organisation (CRO) providing services for a reputed city-based pharmaceutical manufacturer.
“On July 17, five days after I was given the medicine on July 12, I suffered a heart attack, and I believe it was because of the drug I was given,” Dipankar Dey, a centreing worker from Kolkata, who ‘volunteered’ for the trial in return for a promised sum of Rs.25,240.
Dey said on July 17, when he experienced discomfort in his chest he put it to acidity and went to a medical shop near one of the cheap lodges he was staying in opposite Secunderabad railway station, to buy some antacid. He was advised that the discomfort he was experiencing could be related to his heart and was told to go to a hospital.
“I went to a nearby private hospital where the doctors after an ECG and other tests, said I was getting a heart attack and must be admitted,” Dey told Deccan Chronicle.
“I called the company and I was told to go there immediately. They said ‘take an auto and come’. It took me an hour and a half to reach their facility in Balanagar from Secunderabad.”
While he made it clear that the company did not offer to get him an ambulance for him, employees at the CRO took him to a private hospital in Jeedimetla where he was admitted, and underwent ‘thrombolysis’ – the medical procedure for dissolving blood clots — and a stent was placed in one of the heart blood vessels.
“When I asked them for compensation a day after recovering. Three women who said they were from an ethics committee who came to meet me, promised to help. An hour later they came back and asked for a letter saying I was well and healthy. I asked how could I be well after suffering a heart attack a day before but they insisted that they will have to show the letter to ‘them’ for any compensation to come,” Dey said.
Volunteers for a drug trial are expected to sign an ‘informed consent’ form. Every drug trial has its own independent ethics committee.
The member-secretary of the independent ethics committee listed in the informed consent form, when contacted, said that Dey was paid Rs.30,000 as compensation by the company, and that a serious adverse event report was filed with the Central Drugs Standards Control Organisation (CDSCO) soon after their visit to the hospital.
The committee member also said the volunteer had ‘bad habits’ like smoking but did not have a clear response when asked why a volunteer with ‘bad habits’ was included in the trial.
Incidentally, the informed consent form specifically mentions that a participant should be a non-smoker. Dey said that while signing the form, he informed the company staff that he had studied up to Class 8 but was told that he should put Class 12 as his education level to qualify.

